THE INFLAMMATION MODEL
A light bulb uses electrical energy to generate light. But, the electrical energy is not created by the light bulb. The electrical energy is created in a location far away and brought to the light bulb using electrical wires.
Pain is an electrical signal in the brain. The pain electrical signal is brought to the brain by nerves. As a rule (there are exceptions), the brain does a good job at identifying the body region that initiates the electrical signal. The point is that the electrical signal in the brain for pain is created at another location and brought to the brain by nerves.
If one stubs one’s toe with sufficient force to achieve excitation threshold of the toe pain nerves (nociceptors), the brain will receive the appropriate electrical signal. The brain will identify toe tissue as the generator of the electrical signal that the brain interprets as being painful.
Studies looking at whiplash injuries consistently identify the facet joints as the primary source of chronic whiplash injury pain (1, 2, 3). The second most common tissue source of chronic whiplash injury pain is the annulus of the intervertebral disc. Injury to the facet joints (and/or the intervertebral disc) initiates a chemical inflammation which alters the threshold of the joint’s pain afferents (nociceptors). If nociceptive excitation threshold is achieved, a local electrical signal will be initiated and travel to the brain where the signal is interpreted as being painful.
Studies looking at chronic low back pain consistently identify the intervertebral disc as the primary source of the electrical signal that travels to the brain (4, 5). A number of studies have shown that when the human intervertebral disc degenerates, the nociceptive nerve fibers in the annulus can migrate into the nucleus pulposus, allowing the nucleus itself to be the tissue source of the nociceptive electrical signal (6, 7, 8).
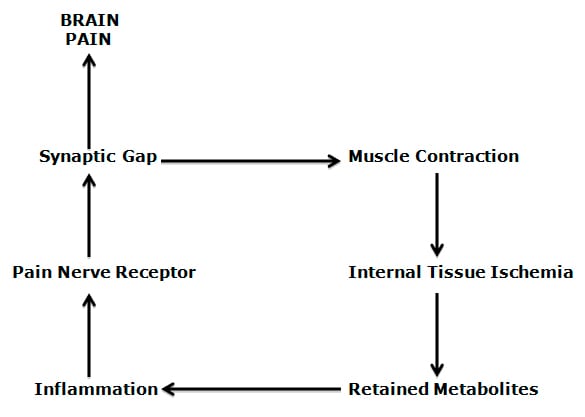
The electrical signal from the injured tissue to the brain is not carried on a single neuron, but rather on a series of neurons. The electrical signal carried from one neuron to next neuron must cross a physical gap, a synaptic gap. The signal is carried across the synaptic gap by chemicals that are produced and released by the first neuron, carried across the synaptic gap to the second neuron where the electrical signal is re-established and continues towards the brain.
The synaptic gap, or synapse is very important because it is a site where the electrical signal to the brain has the potential to be modified (enhanced or reduced).
As a rule, the pain afferents in the facet and/or disc initiate the pain electrical signal as a consequence of being exposed to inflammatory chemicals. In fact, in 2007, Omoigui states (9):
“Irrespective of the type of pain whether it is acute or chronic pain, peripheral or central pain, nociceptive or neuropathic pain, the underlying origin is inflammation and the inflammatory response.”
“Activation of pain receptors, transmission and modulation of pain signals, neuro-plasticity and central sensitization are all one continuum of inflammation and the inflammatory response.”
“Irrespective of the characteristic of the pain, whether it is sharp, dull, aching, burning, stabbing, numbing or tingling, all pain arises from inflammation and the inflammatory response.”
Weiner’s Pain Management, A Practical Guide for Clinicians, by the American Academy of Pain Management, indicates that the primary reason for the pain producing inflammatory cascade is an overabundance of omega-6 fatty acids, acquired from excessive consumption of vegetable oils (10).
It is rational that the treatment of these patients should include anti-inflammatory strategies. Conservatively, proven helpful anti-inflammatory strategies include the local application of ice (11), supplemental omega-3 fatty acids (fish oil) (12, 13, 14), and low-level laser therapy (15).
An important addition to this discussion is the contribution of muscles to pain. Any direct injury to a muscle can cause inflammation and therefore pain. Chronic musculoskeletal pain often has a significant muscle contribution. The muscle contribution is often referred to as Myofascial Pain Syndrome, championed by many, and pioneered by Janet Travell, MD and David Simons, MD (16, 17, 18 19).
A simple explanation of the muscle-pain model is presented by Rene Calliet, MD, and involves these sequential steps (20):
1) Inflammation results in nociceptive threshold, generating an action potential that brings (via the primary afferent) an electrical signal to the spinal cord.
2) In the spinal cord, the primary nociceptive afferent releases chemicals that cross the synaptic gap and excite the second order nociceptive afferent neuron to generate an action potential, moving the electrical signal towards the brain.
3) In the spinal cord, the primary nociceptive afferent also releases chemicals that cross the synaptic gap and generate an electrical action potential in the alpha motor neuron. This causes the alpha motor neuron to increase its production and release of the chemical acetylcholine into the nerve-muscle (myoneuro) junction, causing the muscle to contract (increasing the interdigitation of the contractile proteins). This is the classic afferent-efferent spinal cord reflex. Thus, chronic pain results in chronically contracted muscles. Chronically contracted muscles will cut off its own blood supply, resulting in both internal ischemia and an accumulation of metabolites (waste products). Muscle ischemia and accumulated waste products create an inflammatory response, altering the nociceptive threshold, generating a pain-producing electrical signal to the spinal cord. This is the classic self-perpetuation positive feedback loop, the so-called “pain-muscle-pain” cycle.
It is rational that the treatment of the muscle component of chronic pain syndromes (myofascial pain syndromes) would include ischemic compression (16, 17, 18), spray and stretch techniques (16, 17, 18), needle acupuncture (21) and low-level laser therapy (21).
THE GATE THEORY
The Gate Theory of Pain was first proposed by Canadian psychologist Ronald Melzack, PhD, and British neuroscientist Patrick Wall, PhD (while working at the Massachusetts Institute of Technology) in 1962 in the journal Brain (22). It was not until their work was published in the journal Science in 1965 that it generated widespread attention (23). The Theory, as stated by American neuroscientist and 2000 Nobel Prize winner Eric Kandel (24), is:
“Pain is not simply a direct product of the activity of nociceptive afferent fibers but is regulated by activity in other myelinated afferents that are not directly concerned with the transmission of nociceptive information.”
“The idea that pain results from the balance of activity in nociceptive and non-nociceptive afferents was formulated in the 1960s and was called the gate control theory.”
“Simply put, non-nociceptive afferents ‘close’ and nociceptive afferents ‘open’ a gate to the central transmission of noxious input.”
Simply stated, the Gate Theory of Pain states that pain can be controlled (inhibited) by improving the function of other non-nociceptive nerves. Even though this Theory is half a century old, its principles “have survived the test of time.” (25)
The Gate Theory of Pain has been accepted as a viable explanation for the benefits of chiropractic spinal adjusting for patients with pain syndromes. As noted by Canadian orthopedic surgeon WH Kirkaldy-Willis, MD (26):
“Spinal manipulation is essentially an assisted passive motion applied to the spinal apophyseal and sacroiliac joints.”
Melzack and Wall proposed the Gate Theory of Pain in 1965, and this theory has “withstood rigorous scientific scrutiny.”
“The central transmission of pain can be blocked by increased proprioceptive input.” Pain is facilitated by “lack of proprioceptive input.” This is why it is important for “early mobilization to control pain after musculoskeletal injury.”
The facet capsules are densely populated with mechanoreceptors. “Increased proprioceptive input in the form of spinal mobility tends to decrease the central transmission of pain from adjacent spinal structures by closing the gate. Any therapy which induces motion into articular structures will help inhibit pain transmission by this means.”
Stretching of facet joint capsules will fire capsular mechanoreceptors which will reflexly “inhibit facilitated motoneuron pools” which are responsible for the muscle spasms that commonly accompany low back pain.
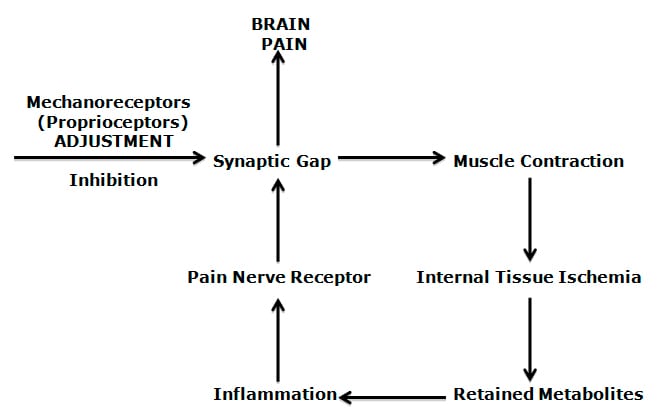
Mechanoreceptors (proprioceptors) are specialized neurons that register the way we live, exist, and function in a gravity environment. These mechanoreceptors are abundant in facet joint capsules, annulus of the disc, paraspinal ligaments, and muscles (27, 28, 29, 30, 31, 32).
Mechanoreceptors are probably the most important source of the “non-nociceptive” afferents that control pain; improving their function closes the pain gate, reducing pain. The increased muscle tone and/or muscle spasm that commonly accompany spinal pain syndromes also reduce spinal motion, reducing articular mechanoreception, opening the pain gate.
The muscles become an important player in chronic spinal pain syndromes because of overlapping feedback loops:
1) Pain increases muscle tone.
2) Increased muscle tone causes inflammation and more pain (a feedback loop).
3) Increased muscle tone reduces articular range of motion.
4) Reduced articular range of motion reduces articular mechanoreception.
5) Reduced articular mechanoreception leaves the pain gate open.
6) An “open” pain gate results in more pain and more muscle tone (again, a feedback loop).
These feedback loops were excellently reviewed by Yale’s Manohar Panjabi, PhD, in 2006 (33):

An important basis for Dr. Panjabi’s model was the animal studies by Aage Indhal, MD, and colleagues from Norway (34). They clearly showed the interrelationships between spinal pain and the contraction of the segmental muscles, reducing the movement parameters of that region of the spine. In later works, Dr. Indahl and colleagues were able to show that by firing the mechanoreceptors of the facet joint capsules they could inhibit both the spinal cord reflex to the segmental muscles and also inhibit pain (35). Thus, by improving mechanical afferentation, they could inhibit both the segmental muscle contraction and inhibit pain (“close” the pain gate).
Dr. Indahl’s experimental models and Dr. Panjabi’s theoretical models are well presented as being applicable to chiropractic spinal adjusting by Raymond Brodeur, chiropractor and engineer working at Michigan State University (36). Dr. Brodeur’s basic premise is that the cavitation associated with the audible adjusting of a spinal segment has sufficient speed to fire off high-threshold mechanoreceptors, inhibiting segmental muscle tone, and improving motion. This would close the pain gate. Dr. Brodeur also makes the point that chiropractic drop tables and Activator adjusting instruments are capable of achieving the same mechanical benefits.
The primary research by Canadian orthopedic surgeon WH Kirkaldy-Willis, MD was clearly able to show that chiropractic spinal adjusting, as monotherapy, was capable of inhibiting both muscle spasm and pain (37). Dr. Kirkaldy-Willis used 283 patients with chronic, severe, treatment resistant low back pain and documented that chiropractic spinal adjusting was able to resolve the clinical syndrome in 81% of the patients. Dr. Kirkaldy-Willis’ explanation for the observed improvement in clinical status was the firing of facet capsule mechanoreceptors, which inhibits the muscle spasm and closes the pain gate.
THE NEWEST MODEL
Catechol-Oxygen-Methyltransferase = Catechol-O-Methyltransferase
It has been known for more than a century that the sympathetic nervous system is involved in chronic pain syndromes. Dr. S. Weir Mitchell and colleagues produced important clinical descriptions of sympathetic pain on injured soldiers during the US Civil War (1861-1865). A search of the US National Library of Medicine using the PUBMED search engine (03/11/14), using the words “sympathetic pain” located 5,597 citations.
Many researchers have observed a psychosocial component to chronic pain syndromes, particularly in the realm of whiplash trauma recovery. A search of the US National Library of Medicine using the PUBMED search engine (03/11/14), using the words “whiplash pain AND psychosocial” located 54 citations.
The 2013 book Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System, shows an impressive picture (my rendition below) of the post-ganglionic sympathetic efferents production and release of the catecholamine norepinephrine to receptors located on the nociceptive afferent axons and dorsal root ganglions (DRG) (38). These catecholamines alter the threshold of the nociceptive afferents so that they more easily reach excitation threshold, more readily producing pain. [As a historical side note, the book’s second author, Sir Roger Bannister, age 84, is about to celebrate his 60th anniversary of being the first human to run a mile in less than 4 minutes, May 6, 1954].
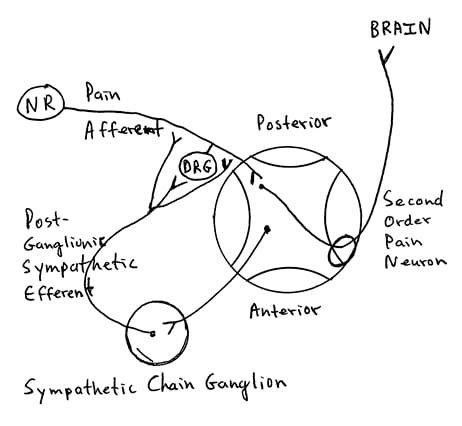
There is mounting evidence that increased sympathetic production and release of catecholamines is a primary driver for both increased chronic pain and for the psychosocial components of chronic pain syndromes, and this phenomenon has a genetic link. This could help explain why so many with chronic pan also have an abnormal psychological profile (39).
Humans have a gene that produces an enzyme that metabolizes sympathetically produced catecholamines. It is called:
Catechol-Oxygen-Methyltransferase = Catechol-O-Methyltransferase
It is abbreviated COMT. Low production of COMT has been linked to increased and chronic pain perception, including temporomandibular pain, fibromyalgia, and whiplash injury pain (40, 41, 42). Samuel A McLean, MD, and colleagues from the University of North Carolina School of Medicine, note (41):
“Genetic variations in the catechol-o-methyltransferase (COMT) gene have been associated with experimental pain and risk of chronic pain development, but no studies have examined genetic predictors of neck pain intensity and other patient characteristics after motor vehicle collision (MVC).”
“These findings suggest that genetic variations affecting stress response system function influence the somatic and psychological response to MVC, and provide the first evidence of genetic risk for clinical symptoms after MVC.”
For decades, Princeton educated physiologist Irvin Korr, PhD, engaged in clinical research showing a link between joint mechanoreceptors (proprioceptors) and sympathetic nervous system production of catecholamines (43, 44). His work showed that improvement of mechanical function not only inhibited pain and muscle spasm (tone), but also inhibited sympathetic activity through a spinal cord reflex. Thus, Dr. Korr would argue that one would have to adjust the correct spinal level, the level of the reduced motion, in order to appropriately inhibit the local increased production of catecholamines.
SUMMARY
The information presented here outlines the physiological explanation for the observation that chiropractic spinal adjusting helps people with chronic or acute pain syndromes. Chiropractic spinal adjusting uses bones as levers to influence the quality of tissue and therefore the integrity of the tissue’s mechanoreceptors. The subsequent improved mechanoreception does the following:
1) Neurologically inhibits the pain electrical signal to the brain. This is synonymous with the “closing” of the pain gate.
2) Neurologically inhibits muscle tone through a spinal cord reflex. The subsequent relaxation of the muscle reduces secondary muscle pain.
3) Neurologically reduces sympathetic tone and the sympathetic nervous system’s production of catecholamines. Since sympathetically produced catecholamines alter the threshold of the primary pain afferent, this mechanically driven inhibition would further inhibit the nociceptive electrical signal to the brain.
REFERENCES
1) Bogduk N, Aprill C; On the nature of neck pain, discography and cervical zygapophysial joint blocks; Pain; August 1993;54(2):213-7.
2) Cusick JF, MD; Pintar FA; Yoganandan N; Whiplash Syndrome: Kinematic Factors Influencing Pain Patterns; Spine; 2001;26:1252-1258.
3) Bogduk N; On Cervical Zygapophysial Joint Pain After Whiplash; Spine; December 1, 2011; Volume 36, Number 25S, pp S194–S199.
4) Mooney V; Presidential address: International Society for the Study of the Lumbar Spine: Dallas, 1986. Where is the pain coming from? Spine; 1987 Oct;12(8):754-9.
5) Kuslich S, Ulstrom C, Michael C; The Tissue Origin of Low Back Pain and Sciatica: A Report of Pain Response to Tissue Stimulation During Operations on the Lumbar Spine Using Local Anesthesia; Orthopedic Clinics of North America, Vol. 22, No. 2, April 1991, pp. 181-187.
6) Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O’Brien J, Jayson MIV; Nerve Ingrowth Into Diseased Intervertebral Disc in Chronic Back Pain; The Lancet; July 19, 1997, Vol. 350, pp. 178-181.
7) Coppes MH, Marani E, Thomeer RT, Groen GJ; Innervation of “painful” lumbar discs; Spine; 1997 Oct 15;22(20):2342-9.
8) Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MT, Ross ER, O'Brien JP, Hoyland JA; Nerve growth factor expression and innervation of the painful intervertebral disc; Journal of Pathology; 2002 Jul;197(3):286-92.
9) Omoigui S; The biochemical origin of pain: The origin of all pain is inflammation and the inflammatory response: Inflammatory profile of pain syndromes; Medical Hypothesis; 2007, Vol. 69, pp. 1169-1178.
10) Boswell M, Cole BE; American Academy of Pain Management, Weiner’s Pain Management: A Practical Guide for Clinicians; Seventh Edition, 2006, pp. 584-585.
11) Kellett K; Acute soft tissue injuries--a review of the literature; Medicine and Science in Sports and Exercise; October 1986;18(5):489-500.
12) Maroon JC, MD, Bost JW; Omega-3 Fatty acids (fish oil) as an anti-inflammatory: an alternative to nonsteroidal anti-inflammatory drugs for discogenic pain; Surgical Neurology; 65 (April 2006); pp. 326–331.
13) Cleland LG, James MJ, Proudman SM; Fish oil: what the prescriber needs to know; Arthritis Research & Therapy; Volume 8, Issue 1, 2006, pp. 402.
14) Goldberg RJ, Katz J; A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain; Pain; May 2007, 129(1-2), pp. 210-223.
15) Tuner J, Hode L; The New Laser Therapy Handbook: A Guide for Research Scientists, Doctors, Dentists, Veterinarians and other Interested Parties Within the Medical Field; Prima Books AB, 2010.
16) Travell J, Simons D; Myofascial pain and dysfunction, the trigger point manual; New York: Williams & Wilkins, 1983.
17) Travell J, Simons D; Myofascial pain and dysfunction, the trigger point manual: THE LOWER EXTREMITIES; New York: Williams & Wilkins, 1992.
18) Simons D, Travell J; Travell & Simons’, Myofascial pain and dysfunction, the trigger point manual: Volume 1, Upper Half of Body; Baltimore: Williams & Wilkins, 1999.
19) Mense S; Simons DG; Muscle Pain: Understanding its Nature, Diagnosis, and Treatment; Lipponcott Williams & Wilkins; 2001.
20) Cailliet R; Soft Tissue Pain and Disability; 3rd Edition; FA Davis Company, 1996.
21) Gunn CC; The Gunn Approach to the Treatment of Chronic Pain: Intramuscular Stimulation for Myofascial Pain of Radiculopthic Origin; Churchill Livingston, 1996.
22) Melzack R, Wall PD; On the nature of cutaneous sensory mechanisms; Brain. 1962 Jun;85:331-56.
23) Melzack R, Wall PD; Pain mechanisms: a new theory; Science; 1965; Nov 19;150(3699):971-9.
24) Eric Kandel, James Schwartz, Thomas Jessell, Principles of Neural Science. McGraw-Hill, 2000.
25) Dickenson AH; Gate Control Theory of pain stands the test of time; British Journal of Anaesthesia, Vol. 88, No. 6, June 2002, pp. 755-757.
26) Kirkaldy-Willis WH; Cassidy JD; Spinal Manipulation in the Treatment of Low back Pain; Canadian Family Physician; March 1985, Vol. 31, pp. 535-540.
27) Mendel T, Wink CS, Zimny ML; Neural elements in human cervical intervertebral discs; Spine; February 1992;17(2):pp. 132-5.
28) McLain RF; Mechanoreceptor endings in human cervical facet joints; Spine; March 1, 1994;19(5):495-501.
29) McLain RE, Pickar JG; Mechanoreceptor endings in human thoracic and lumbar facet joints; Spine; January 15, 1998;23(2):168-73.
30) Roberts S, Eisenstein SM, Menage J, Evans EH, Ashton IK; Mechanoreceptors in intervertebral discs: Morphology, distribution, and neuropeptides; Spine; December 15, 1995;20(24): pp. 2645-51.
31) Dimitroulias A, Tsonidis C, Natsis K, Venizelos I, Djau SN. Tsitsopoulos P; An immunohistochemical study of mechanoreceptors in lumbar spine intervertebral discs; Journal of Clinical Neuroscience; Volume 17, Issue 6, June 2010, Pages 742-745.
32) Holm S, Indahl A, Solomonow M; Sensorimotor control of the spine; Journal of Electromyography and Kinesiology; Volume 12, Issue 3, June 2002, Pages 219-234.
33) Panjabi MM; A hypothesis of chronic back pain: ligament subfailure injuries lead to muscle control dysfunction; Eur Spine J. 2006 May;15(5):668-76.
34) Indahl A, Kaigle A, Reikerås O, Holm S; Electromyographic response of the porcine multifidus musculature after nerve stimulation; Spine (Phila Pa 1976). 1995 Dec 15;20(24):2652-8.
35) Indahl A, Kaigle AM, Reikeräs O, Holm SH; Interaction between the porcine lumbar intervertebral disc, zygapophysial joints, and paraspinal muscles; Spine (Phila Pa 1976). 1997 Dec 15;22(24):2834-40.
36) Brodeur R; The audible release associated with joint manipulation; J Manipulative Physiol Ther. 1995 Mar-Apr;18(3):155-64.
37) Kirkaldy-Willis WH, Cassidy JD; Spinal Manipulation in the Treatment of Low back Pain; Canadian Family Physician; March 1985, Vol. 31, pp. 535-540.
38) Mathias C, Bannister R; Autonomic Failure: A Textbook of Clinical Disorders of the Autonomic Nervous System; Fifth Edition; Oxford University Press; 2013.
39) Wallis BJ, Lord SM, Bogduk N. Resolution of psychological distress of whiplash patients following treatment by radiofrequency neurotomy: a randomised, double-blind, placebo-controlled trial. Pain. 1997 Oct;73(1):15-22.
40) Tchivileva IE, Lim PF, Smith SB, Slade GD, Diatchenko L, McLean SA, Maixner W; Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: A randomized, double–blind, placebo-controlled, crossover pilot study; Pharmacogenet Genomics; 2010 April; 20(4): 239–248.
41) McLean SA, Diatchenko L, Lee YM, Swor RA, Domeier RM, Jones JS, Jones CW, Reed C, Harris RE, Maixner W, Clauw DJ, Liberzon I; Catechol O-Methyltransferase Haplotype Predicts Immediate Musculoskeletal Neck Pain and Psychological Symptoms after Motor Vehicle Collision; J Pain; Jan 2011; 12(1);101-107.
42) Bortsov AV, Diatchenko L, McLean SA; Complex Multilocus Effects of Catechol-O-Methyltransferase Haplotypes Predict Pain and Pain Interference 6 Weeks After Motor Vehicle Collision; Neuromolecular Med. 2014 Mar;16(1):83-93.
43) Korr IM; Proprioceptors and somatic dysfunction; J Am Osteopath Assoc. 1975 Mar;74(7):638-50.
44) Korr IM; The spinal cord as organizer of disease processes: III. Hyperactivity of sympathetic innervation as a common factor in disease; J Am Osteopath Assoc. 1979 Dec;79(4):232-7.